pharmaceutical Technology/Products
WFI Storage Tanks
Working Principles: Water for Injection (WFI) storage tanks are specialized vessels designed for the storage and distribution of highly purified water used in pharmaceutical and biotechnology industries. WFI is a critical component in the production of pharmaceuticals, particularly for formulations, cleaning, and rinsing processes. The working principles of WFI storage tanks involve the following key aspects:
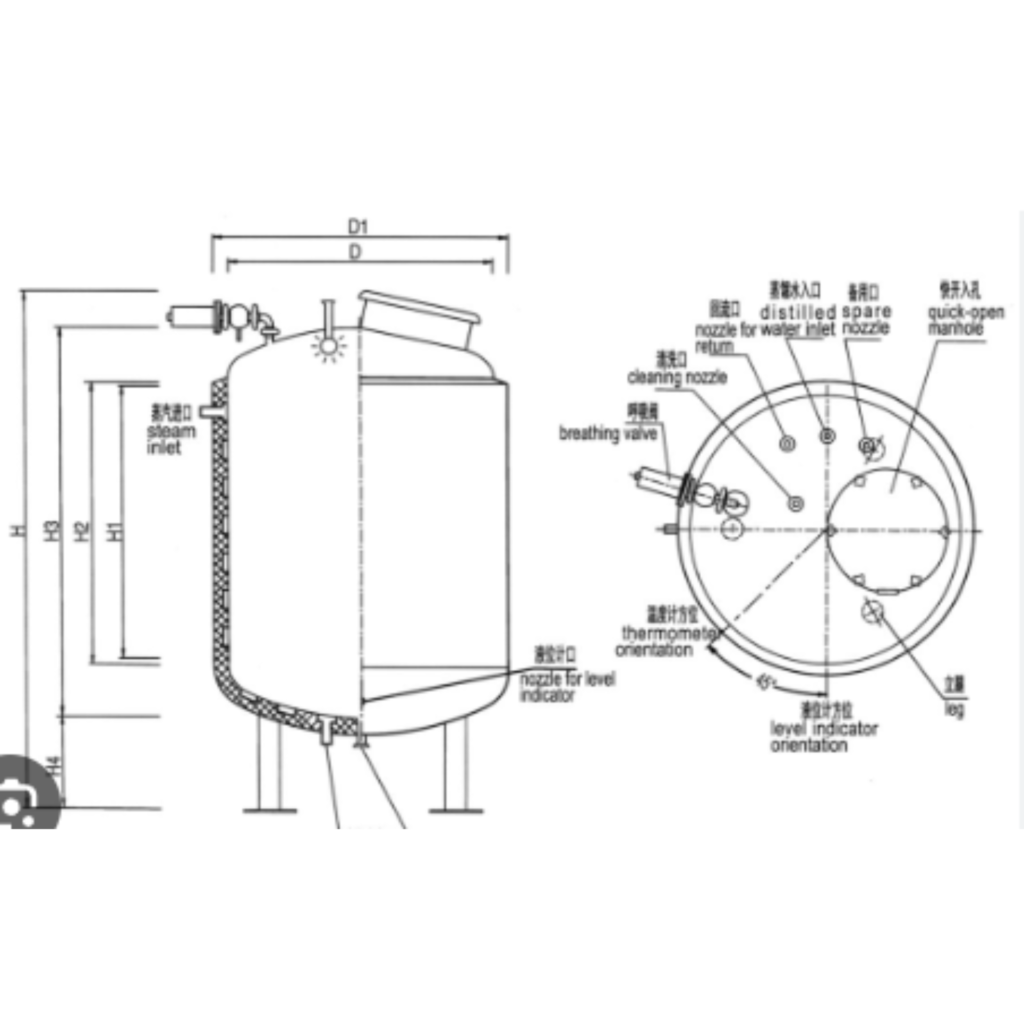
Design: WFI storage tanks are typically constructed from high-quality stainless steel or other materials that are resistant to corrosion and contamination. They are designed with features that ensure the purity and integrity of the stored water.
Purification: The WFI water undergoes a series of purification processes, including distillation, reverse osmosis, and multi-stage filtration, to remove impurities, microorganisms, and endotoxins. This ensures that the water meets the stringent quality standards required for pharmaceutical applications.
Storage: The purified WFI is stored in these tanks under controlled conditions. The tanks are designed to prevent contamination and maintain the water’s quality.
Distribution: WFI storage tanks are equipped with distribution systems that allow for the controlled and aseptic transfer of WFI to various parts of the pharmaceutical manufacturing facility. This distribution system often includes sanitary pumps and piping.
Temperature Control: Temperature control systems are used to maintain the WFI within the specified temperature range, which is typically elevated to prevent microbial growth.

Advantages:
Purity: WFI storage tanks ensure the purity of the water, meeting the stringent quality standards required for pharmaceutical manufacturing.
Consistency: These tanks provide a consistent and reliable source of high-quality water for various processes.
Compliance: WFI storage tanks are designed and operated in compliance with regulatory guidelines, ensuring product safety and adherence to Good Manufacturing Practices (GMP).
Customization: Tanks can be customized to meet specific requirements, including size, capacity, and distribution systems.
Disadvantages:
Cost: The design, construction, and maintenance of WFI storage tanks can be expensive due to the need for high-quality materials and stringent quality control.
Maintenance: Regular maintenance and validation are required to ensure the tanks continue to meet purity standards.
Applications: WFI storage tanks are primarily used in pharmaceutical and biotechnology industries for the following purposes:
Pharmaceutical Formulations: WFI is a critical ingredient in the formulation of injectable drugs, parenteral solutions, and oral liquid formulations.
Cleaning and Rinsing: WFI is used for cleaning and rinsing equipment and containers to maintain sterility and prevent contamination in pharmaceutical manufacturing.
Analytical Testing: High-purity water is required for analytical testing and quality control in pharmaceutical laboratories.

Summary: WFI (Water for Injection) storage tanks are specialized vessels used in the pharmaceutical and biotechnology industries for the storage and distribution of highly purified water. They are designed to maintain the purity and integrity of the stored water, meeting stringent quality standards required for pharmaceutical applications. These tanks ensure the consistency and reliability of the water source for pharmaceutical formulations, cleaning processes, and analytical testing. While they offer advantages in terms of purity and compliance, they come with costs and maintenance requirements to uphold their quality standards.


 Sales & Marketing:
Sales & Marketing:  Service Supports:
Service Supports:  Website:
Website: 