Pharmaceutical Technology/Products

Clean In Place (CIP) is a method used in various industries, including food and beverage, pharmaceuticals, and biotechnology, for cleaning and sanitizing equipment and piping systems without disassembling them.
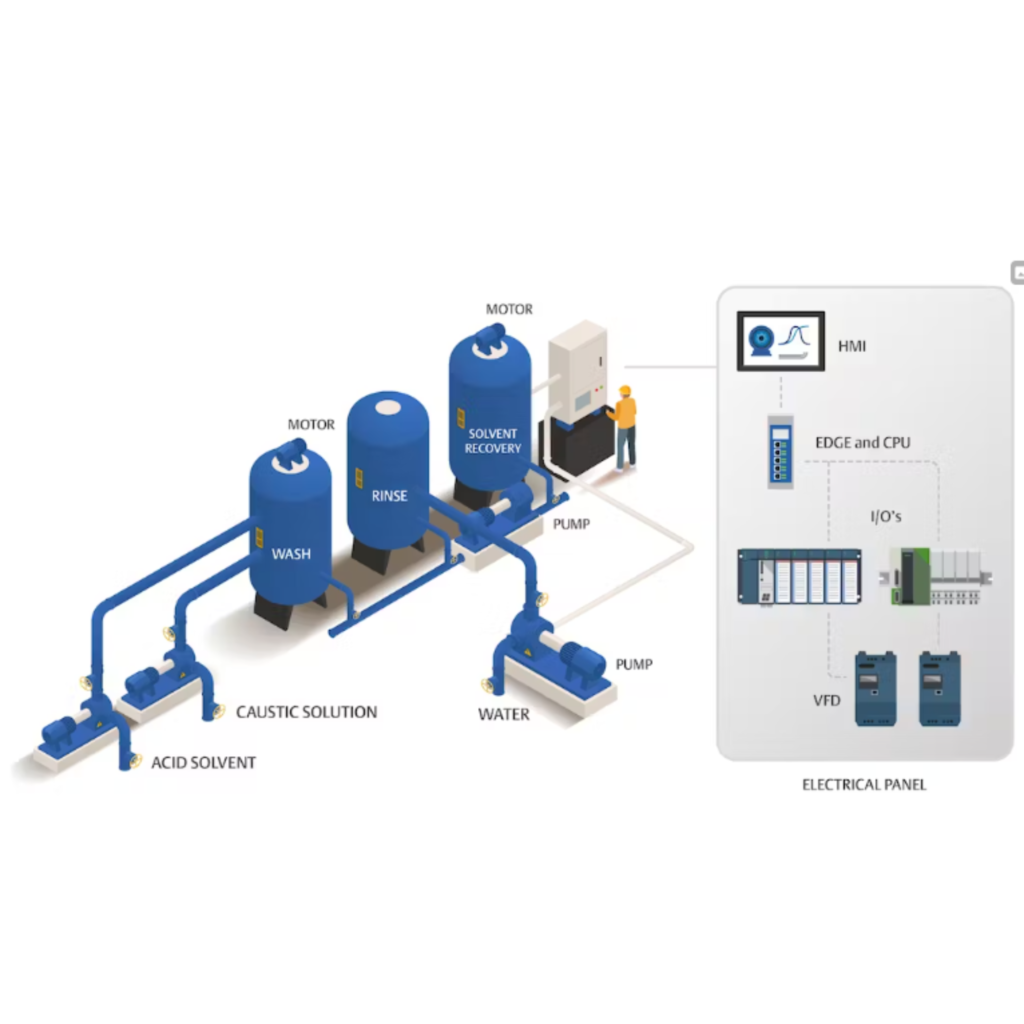
The working principles of CIP involve the following steps:
Preparation: The equipment to be cleaned is identified, and any loose or removable components are removed for manual cleaning if necessary.
Rinse: The CIP system delivers a flow of water or cleaning solution to rinse the equipment’s interior surfaces. The rinse water helps remove loose dirt and contaminants.
Detergent or Cleaning Solution: A detergent or cleaning solution is prepared and circulated through the equipment and piping using pumps and distribution systems.
This solution is formulated to remove residues, oils, fats, and other contaminants.
Agitation: The cleaning solution is agitated within the equipment using mechanical or hydraulic means, ensuring that it comes into contact with all interior surfaces.
Contact Time: The cleaning solution is allowed to remain in contact with the surfaces for a specified time, typically determined by the cleaning process and the nature of the contaminants.
Rinse (Again): After the cleaning solution has completed its cycle, it is flushed from the equipment with a rinse solution (usually water). This removes any remaining cleaning solution and contaminants.
Sanitization (Optional): In some cases, a sanitization step follows, where a sanitizing agent is circulated to kill any remaining microorganisms. This step is crucial in industries with strict hygiene requirements.
Final Rinse: A final rinse with purified or treated water ensures that no cleaning or sanitizing residues are left behind.
Verification: The cleanliness and sanitation of the equipment are verified through visual inspection and testing methods.

Advantages:
Efficiency: CIP systems automate the cleaning process, reducing the need for manual labor and downtime.
Consistency: CIP processes are repeatable and consistent, ensuring a high level of cleanliness and sanitation.
Reduced Risk of Contamination: CIP minimizes the risk of cross-contamination between batches, particularly in the food and pharmaceutical industries.
Time Savings: CIP processes are typically faster than manual cleaning methods.
Disadvantages:
Initial Cost: Implementing CIP systems can be expensive due to the need for specialized equipment and installation.
Complexity: CIP systems require regular maintenance and skilled operators to ensure proper functioning.
Applications: Clean In Place (CIP) is widely used in various industries, including:
Food and Beverage: Cleaning and sanitizing processing equipment, tanks, and pipelines.
Pharmaceuticals: Cleaning and sterilizing equipment used in drug manufacturing.
Biotechnology: Ensuring the cleanliness and sterility of bioreactors and fermentation vessels.
Dairy: Cleaning milk processing equipment and storage tanks.
Brewing: Cleaning brewing vessels and pipes.

Summary: Clean In Place (CIP) is an automated method for cleaning and sanitizing equipment and piping systems without disassembly.
It involves rinsing, using cleaning solutions, agitation, rinsing again, and optionally sanitizing. CIP offers efficiency, consistency, and reduced contamination risk but comes with initial costs and complexity.
It finds applications in industries where maintaining cleanliness and sanitation are critical, such as food and beverage, pharmaceuticals, and biotechnology.


 Sales & Marketing:
Sales & Marketing:  Service Supports:
Service Supports:  Website:
Website: 